Biofuels Research
Personnel:
Dr. Ananda Amarasekara, Group Lead and Co-PI, Department of Chemistry
Objectives and Tasks:
The principal objectives of the biofuels research are to: (1) make in-depth fundamental studies to understand the reaction pathways of fast pyrolysis and how they affect the final composition of bio-oil and other by-products as process variables are varied in an effort to improve yields and quality of specific chemical species present in the bio-oil.; (2) develop an environmentally benign and industrially feasible lignocellulosic biomass hydrolysis method using ionic liquid catalyst for cellulosic-ethanol production, and (3) develop catalytic conversion strategies for upgrading bio-oil to useful fuels.
A. Current Research
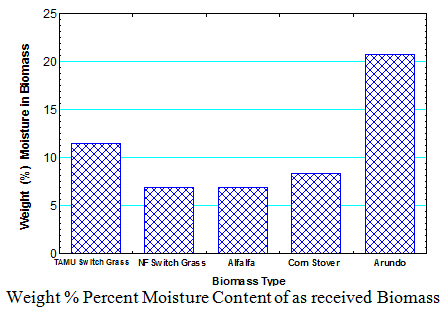
1. Moisture Content of Biomass
The drying procedure used in determining the moisture content was based on NREL LAP on “Preparation of Samples for Compositional Analysis”. The arundo biomass from TAMU had the highest as received moisture content of 20.8%. The alfalfa biomass from the Noble Foundation in Oklahoma had the lowest as received moisture content of 6.4%.
- The high moisture content of arundo and the other biomass make it necessary to dry the biomass in order to reduce the amount of water in the bio-oil produced from these biomass.
- That the moisture content of biomass is highly dependent on the source of the biomass as evidenced by the 11.5% moisture content of the switch grass from Texas A&M and 6.9% moisture content of the switch grass from the Noble Foundation in Oklahoma
2. Soluble Extractives in Biomass
The soluble extractive procedure was based on NREL LAP on “Determination of Extractives in Biomass”, NREL/TP-510-42619 . Three solvents (water, ethanol, and hexane) were used to extract soluble compounds in the various biomass.
The water soluble extractives include: (1) non-structural carbohydrates such glucose, fructose, sucrose, galactose, mannose, arabinose and xylose, (2) alditols such as sorbitol, mannitol and xylitol, (3) organic acids including acetic, lactic, fumaric, malic, citric, hydroxybenzoic and vanillic acid and (4) inorganic ions including Na+, K+, NH4+, Mg2+, Ca2+, Cl–, NO3– and SO42- .
Ethanol soluble extractives include: (1) waxes that consist of fatty acids, fatty alcohols, alkanes, sterols, hydroxyl-beta-diketones and beta-diketones with long carbon chain molecules from 12 up to 38 carbon atoms, (2) fats, (3) tannings, the phenolic class of secondary metabolites in plants, and (4) pigments such as chlorophyll.
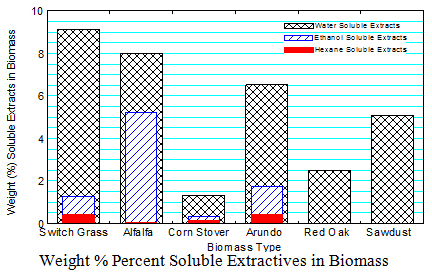
Hexane soluble extractives include (1) highly non-polar waxes, sterol esters, ferulic acid esters, terpenes, furfural alpha- and beta-pinene, and limonene that are not effectively extracted by ethanol. The results indicate that: There are more water soluble extractives in the biomass followed by ethanol soluble extractives. There is very little hexane extractives in the biomass as it followed the ethanol extraction. Switch grass has the highest water soluble extractives (9.1%) and corn stover had the least water soluble extractives (1.3%). Alfalfa has the highest ethanol soluble extracts (5.2%). The highest ethane soluble extracts (0.4%) were in switch grass and Arundo.
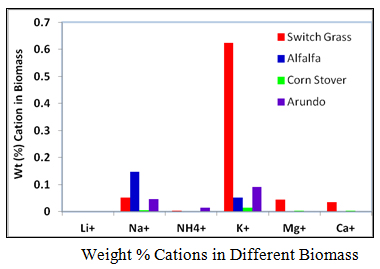
3. Weight (%) Cations in Biomass
The objective of this activity was to perform a compositional analysis on the water-soluble extracts of samples of the five biomass types. The first step was to determine the amounts of cations and anions present in these samples. The knowledge of these ions present will be of utmost importance in the characterization of biomass as they influence the yields of biofuels during the pyrolysis process.
Ion Chromatograph, ICS-5000, for determination of ions in the extracts using the standard operating procedures for using ICS-5000 ion chromatograph for determining anion and cations was used in this work. Three samples were run for each test and the average of the three results used to determine the concentration of ions in the water extracts of the biomass samples using a previously prepared calibration curve with known concentrations of ions as standards. From the volume of extracts and concentration obtained the amounts of ions in the biomass and the composition of cations and anions in each biomass were calculated. Results are shown graphically here.
4. Weight (%) Anions in Biomass
The weight % anions in the biomass obtained from ICS-5000 analysis of the water soluble extractive is shown in the Table below for switch grass an corn stover. This analysis is still in progress for the other biomass.
| Biomass | Wt % Acetate ion |
Wt % ( Cl– |
Wt % ( Br– ) |
Wt % ( NO3– ) |
Wt % ( PO4– ) |
Wt % ( SO4– ) |
| Switch Grass | 0.143 | 0.504 | 0.019 | 0.045 | 0.199 | 0.113 |
| Corn Stover | 0.021 | 0.024 | 0 | 0 | 0.068 | 0.025 |
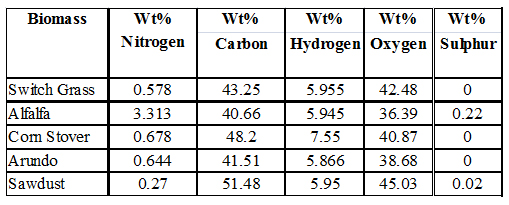
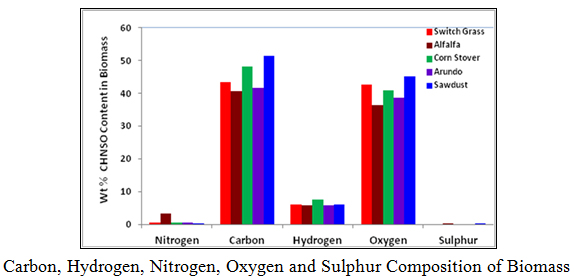
5. CHNSO Elemental Composition of Biomass
The objective of this section of the research is to determine the elemental composition, specifically, carbon, hydrogen, nitrogen, sulphur, and oxygen in switch grass, alfalfa, arundo, corn stover and sawdust. The procedure described in the standard operation procedure for determining CHNS & O composition using the Flash2000 was used in running the tests. The tabulated and graphical results are shown These results are comparable to what are reported in the literature.
6. Biomass Decomposition Kinetics Study Using High Resolution, TA Modulated Q500 TGA
Decomposition kinetics study of the six biomass (alfalfa, switch grass, red oak, corn stover, arundo, and sawdust) have been made using the TA Q500 TGA. The analysis was based on four constant heating rates (2.5, 5, 10 and 20 oC/min) using the conventional TGA approach and analy
![]()
whereα= fraction of decomposition, t = time (seconds), Z = pre-exponential factor (1/seconds), Ea = activation energy (J/mole), R = gas constant (8.314 J/mole K), and n = reaction order (dimensionless). Flynn and Wall rearranged this equation to get
![]()
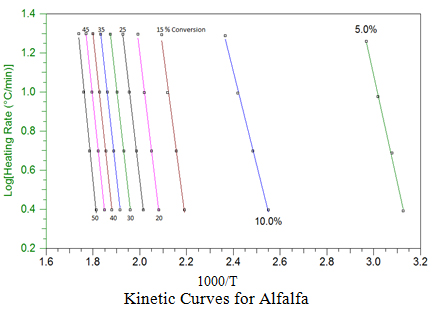
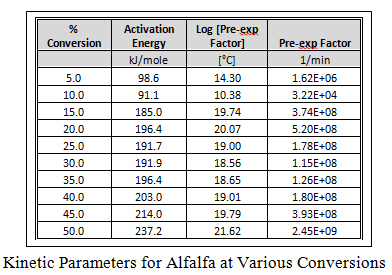
Using a point of equivalent weight loss (decomposition), a plot of ln( b) vs. 1000/T is constructed as shown graphically here for alfalfa at different conversion points. The slopes of these straight line plots are then used to calculate activation energy (Ea) at each conversion, and the pre-exponential factors (Z) are calculated by the interactive method of Zsako and Zsako to generate the kinetic data in the table for alfalfa. This approach was used to generate similar plots and kinetic data table for the other biomass.
7. Research on Development of Brönsted acidic ionic liquids as green catalysts for hydrolysis of lignocellulosic biomass in the cellulosic-ethanol process
Lignocellulosic biomass is composed of cellulose, hemicellulose, and lignin, and the typical percentage composition by dry weight is 35–50% cellulose, 20–35% hemicellulose, and 5–30% lignin. These carbohydrate polymers (cellulose and hemicelluloses) are tightly bound to the lignin, invariably encapsulated in lignin structure.
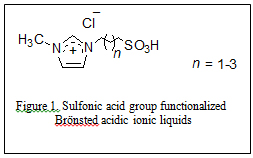
In 2009 our research group introduced the use of sulfonic acid group functionalized Brönsted acidic ionic liquids (figure 1) for concurrent dissolution and hydrolysis of cellulose under mild conditions. In these experiments cellulose (DP ~ 450) was found to dissolve up to 20% w/w in 1-(1-propylsulfonic)-3-methylimidazolium chloride and could be hydrolyzed into glucose and other reducing sugars at 70 °C and atmospheric pressure in excellent yields by treatment with controlled small amount of water (Ind. & Eng. Chem. Res., 2009, 48(22), 10152). The mechanism proposed for the acidic ionic liquid catalyzed hydrolysis of cellulose is shown in figure 2.
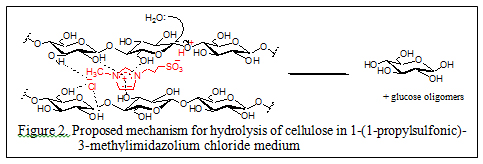
As these neat ionic liquid based cellulose depolymerization methods require large volumes of ionic liquids as solvents and complete recovery and reuse of the ionic liquid is essential in any large scale industrial process. This is quite challenging since both the resulting sugars and these ionic liquids are highly soluble in water. Therefore, in the second stage of our investigations we concentrated our efforts in the direction of using acidic ionic liquid as a glycosidic bond hydrolysis catalyst in aqueous medium. This is a highly attractive proposition and can be seen as an attempt to develop an “artificial cellulase” type green catalyst for the long awaited cellulosic-ethanol process.
Our preliminary efforts in the use of dilute aqueous solutions of acidic ionic liquids in water has shown promising results and some of these results have been published recently (Ind. & Eng. Chem. Res., 2011, 50). In these experiments, we have found that dilute aqueous solutions of 1-(1-propylsulfonic)-3-methylimidazolium chloride and p-toluenesulfonic acid are better catalysts than aqueous sulfuric acid of the same H+ ion concentration for the degradation of cellulose at moderate temperatures and pressures. For example, Sigmacell cellulose (DP ~ 450) in aqueous solutions of 1-(1-propylsulfonic)-3-methylimidazolium chloride, p-toluenesulfonic acid, and sulfuric acid of the same acid strength (0.0321mole H+ ion /L) produced total reducing sugar (TRS) yields of 28.5, 32.6, and 22.0 % respectively, after heating at 170 °C for 3.0 hr. A typical set of TRS % yields versus temperature plots for acidic ionic liquid (1), p-toluenesulfonic acid and sulfuric acid mediums are shown in figure 3.
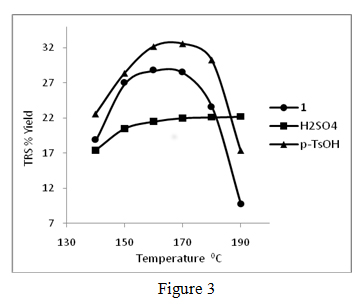
The changes in % yields of total reducing sugar (TRS) produced during the hydrolysis of Sigmacell cellulose (DP ~ 450) in aq.1-(1-propylsulfonic)-3-methylimidazolium chloride, aq. sulfuric acid and aq. p– toluenesulfonic acid at different temperatures. All acid solutions are 0.0321 mol H+/L, reaction time: 3.0 hr., 0.030 g of Sigmacell cellulose in 2.00 mL of aq. acid was used in all experiments. Averages of duplicate experiments. We are continuing these efforts to develop an aqueous phase cellulose hydrolysis catalyst based on ionic liquid core structures, and to produce an artificial cellulase type catalysts for biomass processing. In pursuing this goal we are studying quantitative structure activity relationships (QSAR) in order to understand the mechanisms of interaction of ionic liquid structures like imidazolium cation, and their counter-ions with cellulose in aqueous medium.
B. Research Planned for the Near Future
A number of research activities described below are planned for summer and Fall 2013.
1. Fabrication, Testing and Use of High Conversion Fixed Bed Pyrolysis Reactor
The research team has designed a new fixed bed reactor capable of increasing the conversion rate of biomass to bio-oil. The unique features of this new rector design are the way the career nitrogen gas is introduced and dispersed in the reactor and the way the reaction gases are collected and carried away from the reactor in a way that decreases the residence time in contact with the reactant biomass or residual char. Parts for building the reactor are currently being ordered. The system will be constructed, tested and used to study the effects of processing parameters on biofuel yields with and without catalysts.
2. Determination of structural carbohydrates and Lignin in Biomass
The water, ethanol and hexane extracted biomass will be dried to constant weight and 0.30 g added to a tarred pressure tube. A 3.0 ml of 72% w/w sulfuric acid will be added to the biomass in the pressure tube and the contents thoroughly mixed, placed in a water bath at 30 oC and incubated for 60 min to hydrolyze the biomass. The acid will be diluted 4% w/w and the tube placed in an autoclave safe rack and autoclaved for 1 hour at 121 oC. The hydrolyzate will be allowed to cool slowly after autoclaving and the hydrolysis solution vacuum filtered through a previously weighed filtering crucible. 50 ml of the filtrate will be collected and stored for determination of hydrolyzed structural carbohydrates and acid soluble lignin using the ICS 5000 and a UV-Vis spectrophotometer at 198nm respectively. The acid insoluble lignin will be found by difference after drying the filtered insoluble residue to constant weight.
3. Bio-oil Upgrading into Transportation
The initial step in this task to be initiated this summer will involve screening of catalysts including sulfided Co-Mo-P catalysts, zeolites and commercial ReUSY containing Re2O3 in catalytic conversions of bio-oil by hydro treatment, and simultaneous dehydration and decarboxylation Determining the kinetics and evaluating the yields and selectivity of each catalyst for hydrocarbons and optimizing efficiency of bio-oil conversion is also planned for this Fall.
8. Fermentation of ionic liquids hydrolyzed lignocellulosic biomass fermentable sugars
Determination of the maximum non-inhibitory level of ionic liquid catalysts in fermentable sugars from hydrolysis of lignocellulosic biomass for ethanol fermentation will be conducted using the formulated acidic ionic liquids..
Following this, batch fermentations by recombinant yeast (Saccharomyces cerevisiae TMB3665) expressing pentose utilization pathway and recombinant pentose-fermenting bacterium Zymomonas mobilis AX101 of the fermentable sugars from ionic liquids hydrolyzed lignocellulosic biomass will be conducted.
Participating Researchers and Collaborators: The objectives will be achieved through internal collaborative research. External collaborators from the Biorenewable Institute at Iowa State (ISU) will assist in the research activities at the CREST Center in an advisory and consultation role. (1) ISU will provide distance education course materials (“Fundamentals of Biorenewable Resources” and “Thermochemical Processes”) to PVAMU faculty for the purposes of course development at PVAMU; (2) Summer research experiences for PVAMU faculty and students with support from the CREST center; (3) Research collaborations in biorenewables between ISU and PVAMU faculty; (3) Participation of ISU faculty as members of Program of Study committees for PVAMU graduate students; (4) Offer PhD study opportunities for qualified applicants who complete M.S. degrees at PVAMU (which does not have a PhD program currently) in an effort to increase the number of highly trained minorities in renewable energy research. Texas A&M University in College Station, Texas will assist in shared facilities and assist in securing biomass for the research in addition to Noble Foundations agreement to assist in securing biomass for the research and sharing of research results.
